Petroleum, also known as crude oil, is a naturally occurring liquid composed of hydrocarbons and other organic compounds. It’s found in underground reservoirs and can be extracted through drilling. Petroleum is a vital resource that is used for various purposes such as fueling vehicles, heating homes, and producing plastics, fertilizers, and other materials. However, before it can be used, crude oil must undergo the refining process to separate it into its different components.
The process of fractional distillation
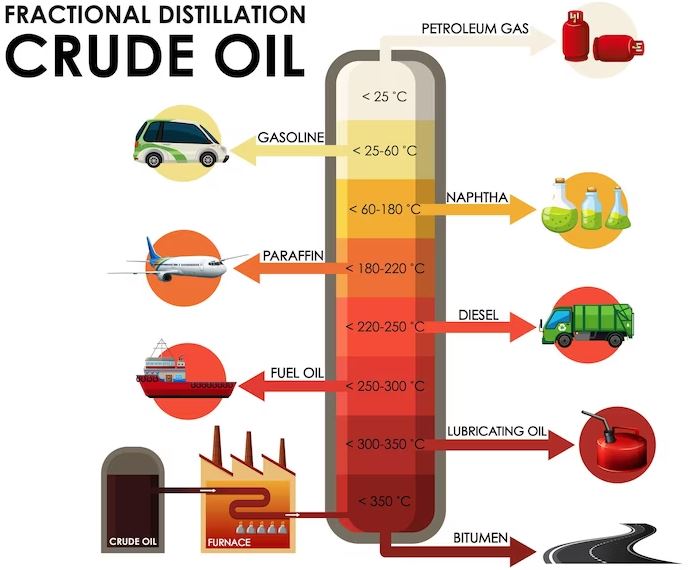
Fractional distillation is the process of separating crude oil into its various components based on their boiling points. This process is carried out in large distillation towers, also known as fractionating columns. The tower is divided into several sections, with each section having a different temperature. The temperature gradually decreases as you move up the tower. The crude oil is heated to a high temperature and then pumped into the bottom of the tower. As the oil rises through the tower, it passes through each section and is cooled by the surrounding air or water.
As the crude oil moves up the tower, it begins to vaporize and separate into different fractions. The lighter fractions, such as gasoline and propane, have lower boiling points and are collected at the top of the tower. The heavier fractions, such as diesel and lubricating oil, have higher boiling points and are collected at the bottom of the tower. This process of separation is based on the principle that different compounds have different boiling points, allowing them to be separated by heating and cooling.
Distillation towers and their components
Distillation towers are the heart of the fractional distillation process. These towers are made up of several components that work together to separate the crude oil into its different fractions. The main components of a distillation tower include the following:
- Heat source: A heat source is required to heat the crude oil to its boiling point.
- Reflux drum: This drum collects the condensed liquid that falls back down the tower, preventing it from being lost.
- Bubble cap trays: These trays are used to distribute the crude oil evenly across the tower. They also prevent the liquid from falling too quickly through the tower.
- Condensers: These are used to cool the vaporized crude oil, turning it back into a liquid.
- Reboilers: These are used to heat the liquid that falls back down the tower, turning it back into a vapor.
Separation of crude oil into fractions
The separation of crude oil into fractions is based on the principle that different compounds have different boiling points. When crude oil is heated, it vaporizes and rises through the distillation tower. As it rises, it cools and condenses into liquid form, with the heavy fractions condensing first and the lighter fractions condensing last.
The first fraction to condense is the heavy residue, which is collected at the bottom of the tower. This residue is used to produce heavy fuels like heating oil and bunker fuel. The next fraction to condense is the gas oil, which is used to produce diesel fuel and other heavier fuels. The lighter fractions, such as naphtha and gasoline, are collected at the top of the tower.
Properties of different petroleum fractions
Each fraction of crude oil has different properties that make it suitable for various purposes. For example, gasoline is a light fraction that has a low boiling point and is highly flammable. It’s used as a fuel for cars and other vehicles. Diesel, on the other hand, is a heavier fraction that has a higher boiling point and is less flammable than gasoline. It’s used as a fuel for trucks and other heavy vehicles.
Lubricating oil is another fraction of crude oil that has a high viscosity, making it ideal for use as a lubricant in engines and machinery. Naphtha is a light fraction that is used as a solvent in the chemical industry. It’s also used as a feedstock for producing petrochemicals like plastics and synthetic rubber.
Uses of petroleum fractions
Petroleum fractions have a wide range of uses in various industries. Gasoline is used as a fuel for cars and other vehicles. Diesel fuel is used in trucks, buses, and other heavy vehicles. Heating oil is used to heat homes and buildings. Lubricating oil is used to lubricate engines and machinery. Naphtha is used as a solvent in the chemical industry and as a feedstock for producing petrochemicals like plastics and synthetic rubber.
Challenges and limitations of fractional distillation
Fractional distillation is a widely used method for refining crude oil, but it has its limitations. One of the main challenges is that it’s difficult to separate some of the heavier fractions, such as bitumen, from the crude oil. These fractions require additional processing, which can be expensive and time-consuming. Another challenge is that the refining process produces a significant amount of waste, including carbon dioxide and other greenhouse gases.
Innovations in fractional distillation
In recent years, there have been several innovations in the field of fractional distillation that aim to improve the efficiency of the process and reduce its environmental impact. One of these innovations is the use of computer modeling to optimize the design of distillation towers. This allows for more precise control of the temperature and pressure within the tower, resulting in better separation of the crude oil into its different fractions.
Another innovation is the use of advanced materials in the construction of distillation towers. These materials are more resistant to corrosion and erosion, reducing the need for maintenance and repair. There has also been research into using renewable energy sources, such as solar and wind power, to generate the heat required for the distillation process.
Conclusion
Fractional distillation is a crucial process in the refining of crude oil. It allows for the separation of crude oil into its different components, which are then used to produce a wide range of products, from gasoline and diesel fuel to plastics and other materials. While the process has its limitations and challenges, innovations in the field aim to improve its efficiency and reduce its impact on the environment. As we continue to rely on petroleum for our energy needs, fractional distillation will remain a vital part of the oil refining industry.


